Evaluating Yield Response of Biological Seed Treatments in Soybean – 2022 Illinois Project Update
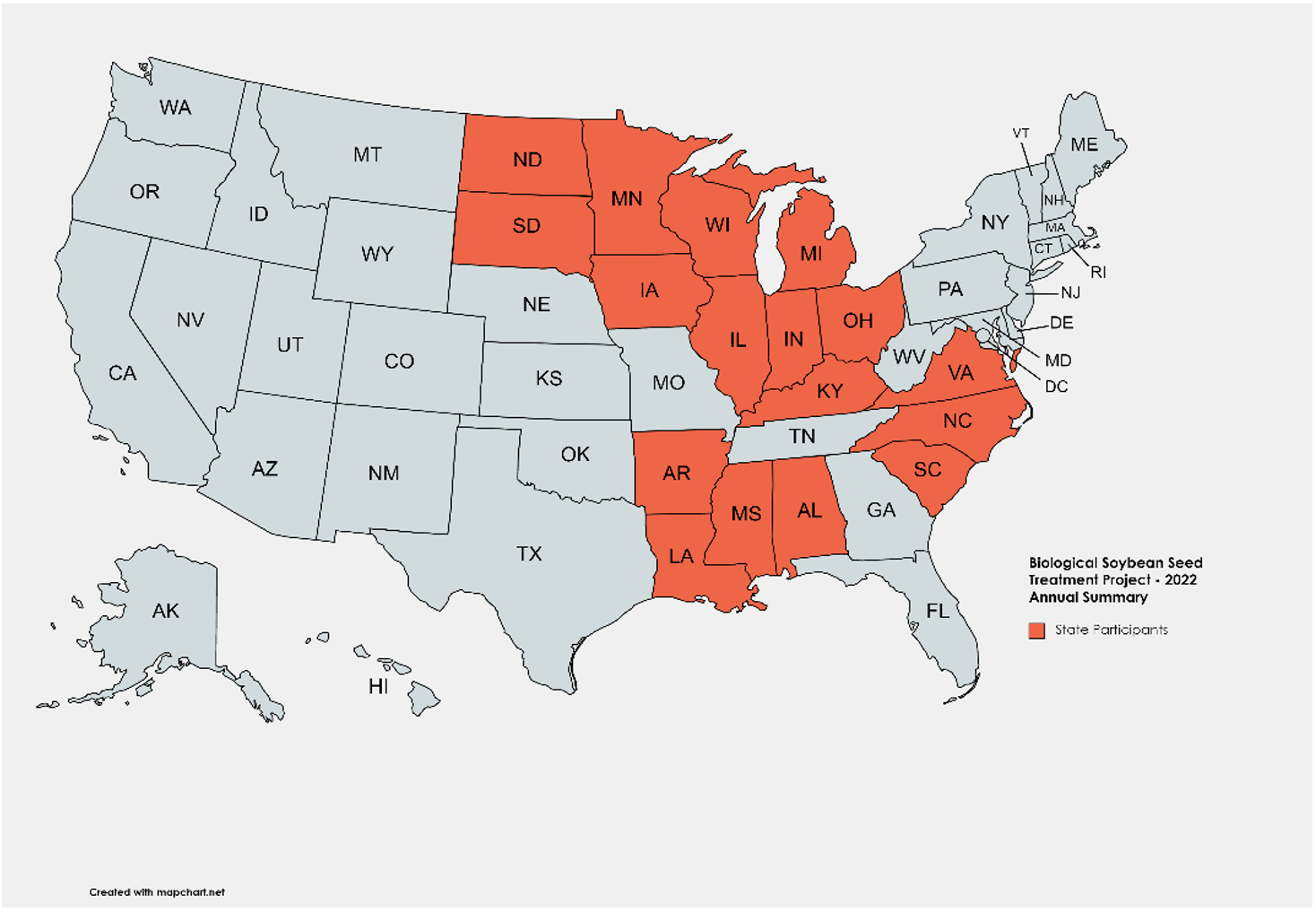
Biological seed treatment is a growing market in the U.S., with several new products being available to growers every year. These products are marketed to increase grain yield and return on investment by the action of a single or a mix of plant-beneficial microbes (e.g., arbuscular mycorrhizal fungi, N-fixing bacteria, and P-solubilizing microbes). Given high commodity prices, growers are interested in understanding the benefits of applying biological products to the seed. In 2022, we evaluated nine commonly marketed biological seed treatment products at two locations in Illinois. In addition, we are part of a national effort to screen commercially available biological seed treatments for soybeans (Figure 1).
Methods
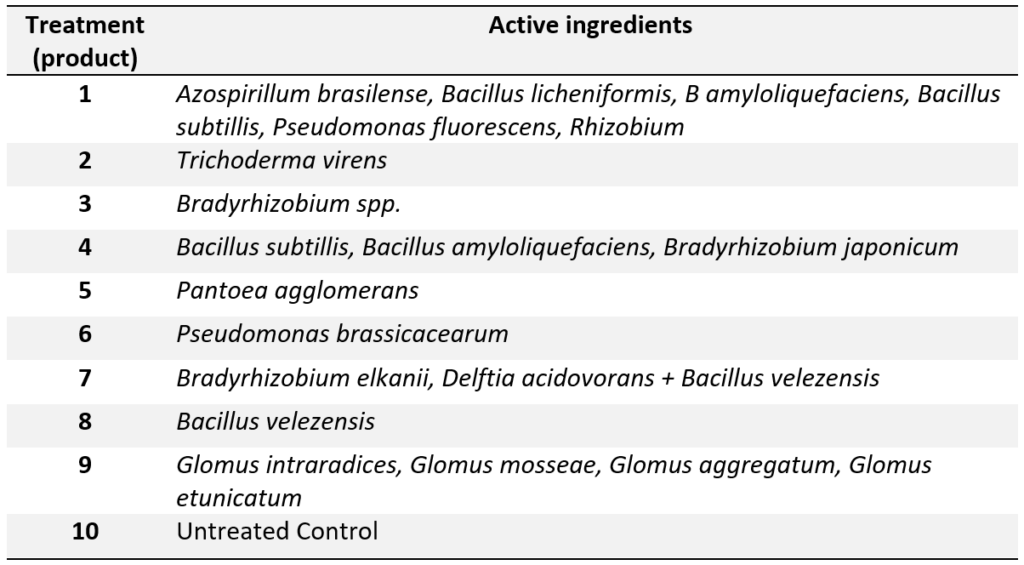
Small-plot trials were located at the University of Illinois Crop Sciences Research & Education centers in Monmouth and Urbana, alongside the soybean variety testing trials (Table 1). All plots were planted with 30-inch-row spacing using a research plot planter at 140,000 seeds per acre. All seeds were pre-treated by the company with fungicide + insecticide. Soybean was grown following standard management practices for weed control and tillage. Harvesting was done using a small-plot combine. Products were applied to the seeds using a small batch seed treater according to the product label. Plant population at growth stage V2 and grain yield were recorded. Seed treatment information for each treatment (product) is presented in Table 2.

2022 Results
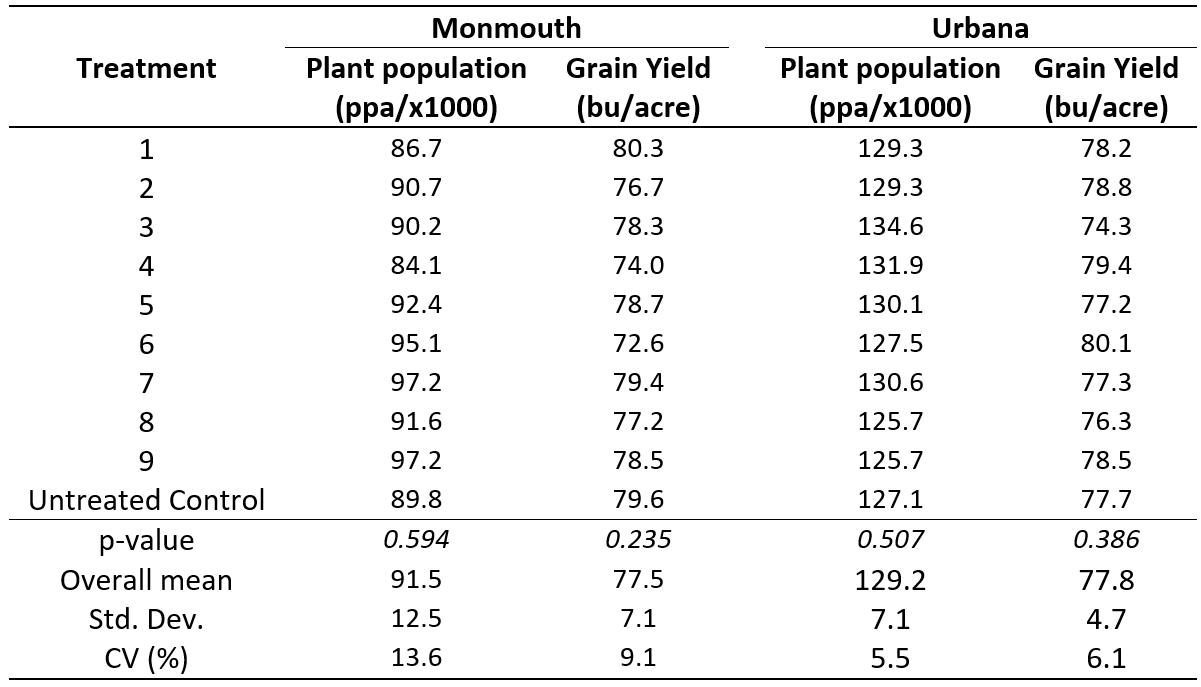
Plant population and grain yield data were analyzed within each location. We found no evidence to conclude that there are statistically significant differences between treatment means (i.e., the p-value is greater than 0.1). A summary of the average plant population and grain yield for each treatment is shown in Table 3.
This study will be repeated in 2023, and Illinois data will be included in a region-wide, multistate and multiyear analysis of yield and population data that will be published in 2024.
This is a preliminary report meant to relay preliminary findings. More data will be released once the trial is complete. This data is not for publication.





